Anti-Aging
Cellular Aging and Telomeres
What are Telomeres?
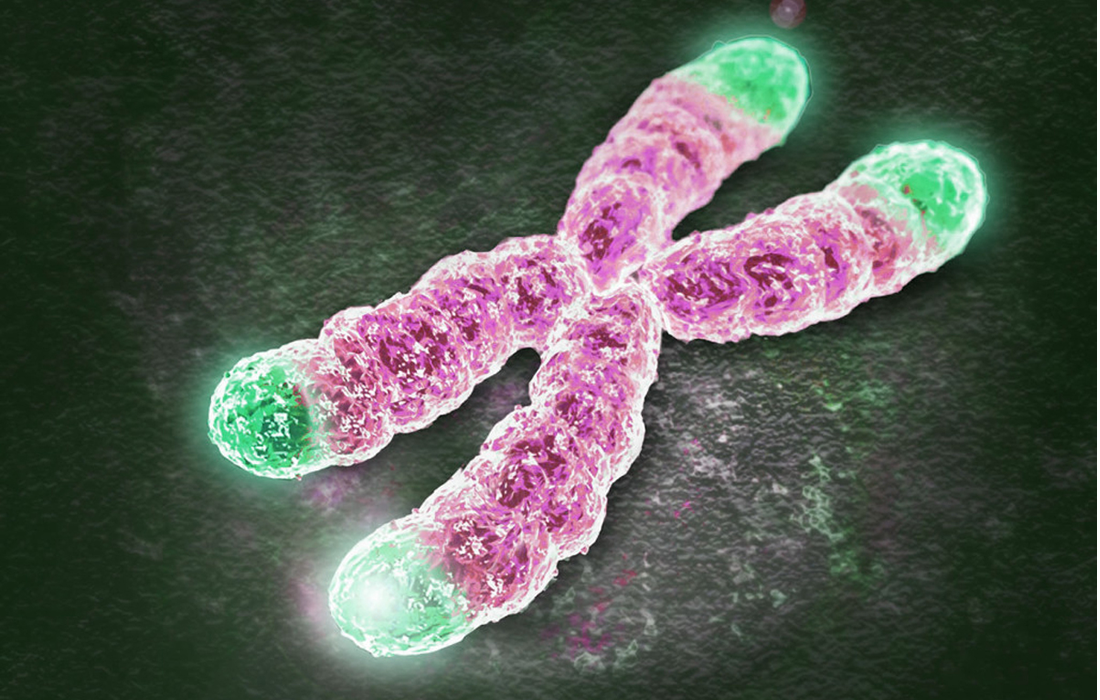
Telomeres are non-coding repetitive DNA sequences at the ends of chromosomes that protect DNA ends from degradation by exonucleases or end-to-end fusions.
When a cell divides, its chromosomes, the bundles of DNA that encode genes, get a little shorter. This is because the cellular machinery for duplicating DNA cannot copy the molecule to the very ends of each strand. To prevent vital genetic information from being lost every time a cell divides, the telomeres protect the ends of the chromosomes, as shown in the image.
These are strips of expendable DNA that don’t encode any important information. However, they erode with every cell division until they can no longer protect the chromosome. At this point, a control mechanism kicks in that stops the cell from dividing any further. Although the cell remains alive and active, it enters into senescence. As we age, the body accumulates these senescent cells and they can promote inflammation, which researchers think underlies many diseases of aging.
New Research Study
A new study published in the journal Nucleic Acids Research helps to reveal how these telomeres trigger cell senescence and cause these age-related diseases. The study was performed at the Université de Montréal in Montreal, Canada. After studying cultures of human skin in their lab.
Scientists claim that cells only stop dividing after chromosomal instability due to loss of telomeres has resulted in irreparable genomic damage during cell division. This updates the previous model that proposes that cells stop dividing simply because their telomeres become too short and no longer function.
One of the senior authors, Francis Rodier, Ph.D. said: “What’s most surprising is that before really entering senescence, the cells divide one last time.” “In fact, the cell division caused by telomere dysfunction is so unstable that it ends up creating genetic defects.”
He also added: “Contrary to what was believed, senescent cells have an abnormal genome.”
Their theory suggests that a single damaged telomere should be sufficient to shut off cell division.
The research may have implications for preventing age-related disease.
In their paper, the scientists conclude: “Paradoxically, our work reveals that senescence-associated tumor suppression from telomere shortening requires irreversible genome instability at the single-cell level, which suggests that interventions to repair telomeres in the pre-senescent state could prevent senescence and genome instability.”
The researchers think that it is possible that therapies aiming at protecting telomere ends before fusions occur would prevent genomic instability and senescence. But some problems remain, such as the fact that preventing a telomere shortening could promote the development of cancer. This is because cancer cells can attain “immortality” by upregulating telomerase.
Sources:
Sabrina Ghadaouia, et al. Homologous recombination-mediated irreversible genome damage underlies telomere-induced senescence, Nucleic Acids Research, 2021;, gkab965, https://doi.org/10.1093/nar/gkab965.
James Kingsland. (2021, Nov 16). Study sheds light on the cellular machinery of aging. Medical News Today. Retrieved from:
https://www.medicalnewstoday.com/articles/study-sheds-light-on-the-cellular-machinery-of-aging
Images from:
Figure 1: Chromosome, telomere and telomerase shown together. The image was made by the Chopra Foundation: https://www.choprafoundation.org/education-research/past-studies/seduction-of-spirit-meditation-study/