Regenerative Medicine News and General Information
First Stem Cell Drug Approved
A stem cell drug was approved for market authorization for the first time in history. Prochymal (remestemcel-L) is also the first drug approved for the treatment of acute graft vs host disease (GvHD) in children.
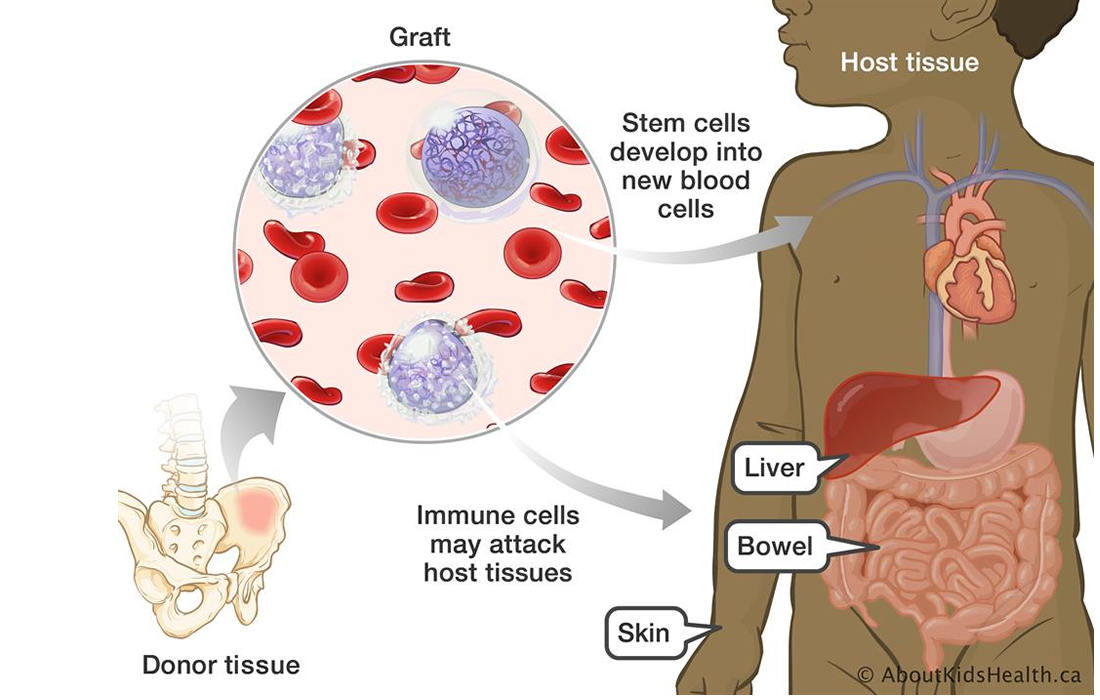
GvHD is a devastating complication of bone marrow transplantation that kills almost 80% of all affected children, many of which just weeks after they have been diagnosed. GvHD is the leading cause of transplant related mortality, caused by an immunologic attack.
Severe GvHD can cause blistering of the skin, intestinal hemorrhage and liver failure, which is extremely painful with a death rate of up to 80%. At present, the first-line standard therapies for GvHD are steroids. Given that the success rate of steroids is only 30%-50%, the only other therapy if steroids fail is limited to immunosuppressive agents that are used off-label with little benefit and significant toxicities. Until the approval of Prochymal, there has not been any other therapy for GvHD.
The company Osiris Therapeutics Inc. was awarded authorization under Canada’s notice of compliance with conditions. This is an authorization to market a drug with the condition that the manufacturer undertakes additional studies to verify the clinical benefit.
Prochymal is an intravenous formulation of mesenchymal stem cells (MSCs) derived from the bone marrow of healthy adult donors aged between 18 to 30 years. They are selected and grown in culture, producing up to 10,000 doses from a single donor. It is stored frozen until it is needed, and infused through a simple IV line without the need to induce immunosuppression in the patient.
Health Canada granted the Prochymal’s authorization for the management of acute GvHD in children who are unresponsive to steroids, based on the drug’s results of clinical trials. The trials demonstrated that in 61%-64% of children with acute GvHD who were unresponsive to steroids, Prochymal produced a clinically significant response at 28 days after the start of the therapy.
Studies continue being made to evaluate the safety and efficacy of the drug. At the moment its use is approved only in Canada and New Zealand.
Source:
https://www.medicalnewstoday.com/articles/245704#5