Regenerative Medicine News and General Information
New Approved Treatment for Vitiligo
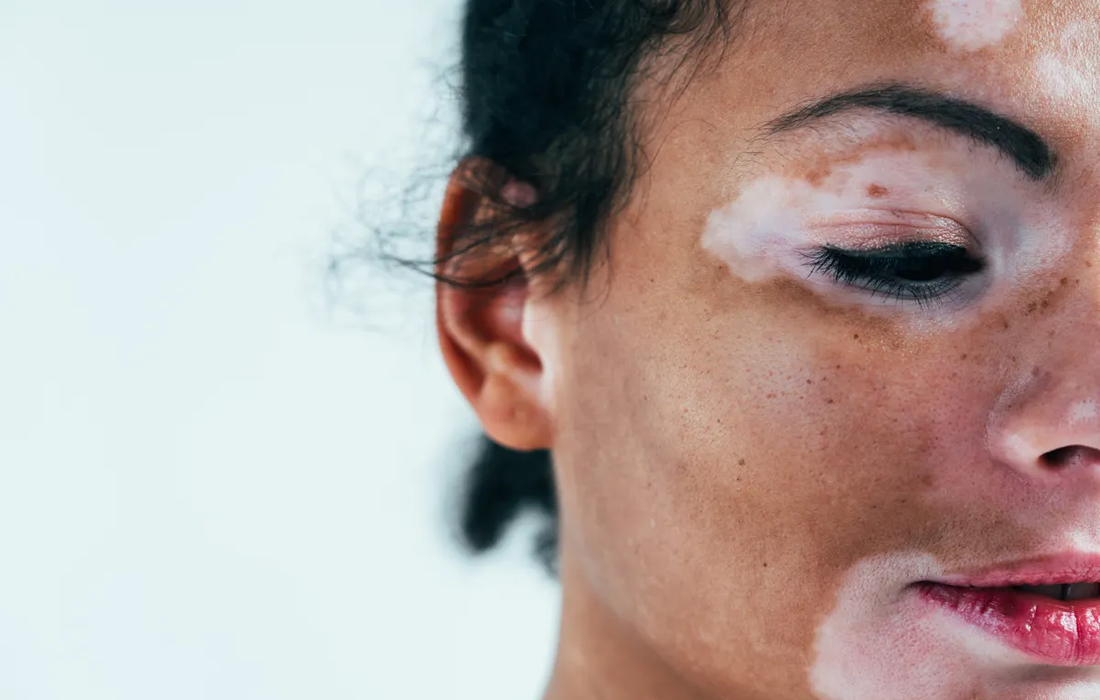
Vitiligo is an acquired pigmentary disorder of the skin that is characterized by circumscribed, depigmented macules and patches. The condition is frequently associated with disorders of autoimmune origin, with thyroid abnormalities being the most common.
Initial lesions occur most frequently on the hands, forearms, feet, and face, favoring a perioral and periocular distribution. The lesions begin as a white or depigmented macules and patches, usually well demarcated; round oval or lineal shape, convex borders, range millimeters to centimeters in size and enlarge centrifugally.
The visual nature of the disease results in severe psychosocial and psychological consequences, especially in individuals with darker skin. It can result in social stigma and depression.
What is the good news for this condition?
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a US Food and Drug Administration (FDA)-approved, easy-to-use topical treatment.
In July, a cream formulation of ruxolitinib , a Janus kinase (JAK) inhibitor, became the first repigmentation treatment approved by the FDA for nonsegmental vitiligo, the most common form of the disease.
David Rosmarin, PhD Dermatologist and his crew, investigate the use of this topic medication for vitiligo disease, they did a multicentre, randomized, double-blind, phase 2 study for adult patients with vitiligo in 26 US hospitals and medical centers in 18 states. Patients with depigmentation of 0·5% or more of their facial body surface area (BSA) and 3% or more of their non-facial BSA were randomly assigned by use ruxolitinib cream or vehicle (control group) twice daily on lesions constituting 20% or less of their total BSA for 24 weeks, and hey increased the dose up to 52 week.
They found good outcomes, if patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back. Half the patients will get 75% or more pigment back in the face.
However, anatomic region matters, skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
Does the medication have any health risks?
There are two main side effects, Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions such as pruritus.
Patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments. One of those is with phototherapy, but a larger study looking at the combination to see whether it is synergistic or not is necessary.
SOURCE:
David Rosmarin, Amit G Pandya, Mark Lebwohl, Pearl Grimes, Iltefat Hamzavi, Alice B Gottlieb, Kathleen Butler, Fiona Kuo, Kang Sun, Tao Ji, Michael D Howell, John E Harris. (July 11, 2020). Ruxolitinib cream for treatment of vitiligo: a randomized, controlled, phase 2 trial. National Library of Medicine. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/32653055/