Regenerative Medicine News and General Information
New Method for Remote Automation of the Growth of Cerebral Organoids
Cell culture has been a principal model for studying human disease and development for over 70 years since the isolation of HeLa cells from a human cervical cancer biopsy. Originally used as a vehicle to research viruses, human cell culture protocols were optimized for quick and easy growth to produce large quantities of material. Automated microfluidics allows us to move beyond traditional protocols by feeding at rates and precision not manually feasible.
Human embryonic stem (hES) cells and induced pluripotent stem (iPS) cells, collectively referred to as pluripotent stem cells (PSCs), have the potential to differentiate into most cell types of the body, and protocols capitalized upon this to generate 3D culture models for human tissues including brain, gut, liver and breast. These self-assembling, organ-specific cell cultures, called organoids, are broadly utilized as in vitro models in developmental research, pathogenesis, and medicine.
The ability of PSC-derived organoids to self-assemble and generate many tissue-specific cell types makes them particularly useful for modeling complex tissues and systems. The brain contains some of the highest complexity in the human body.
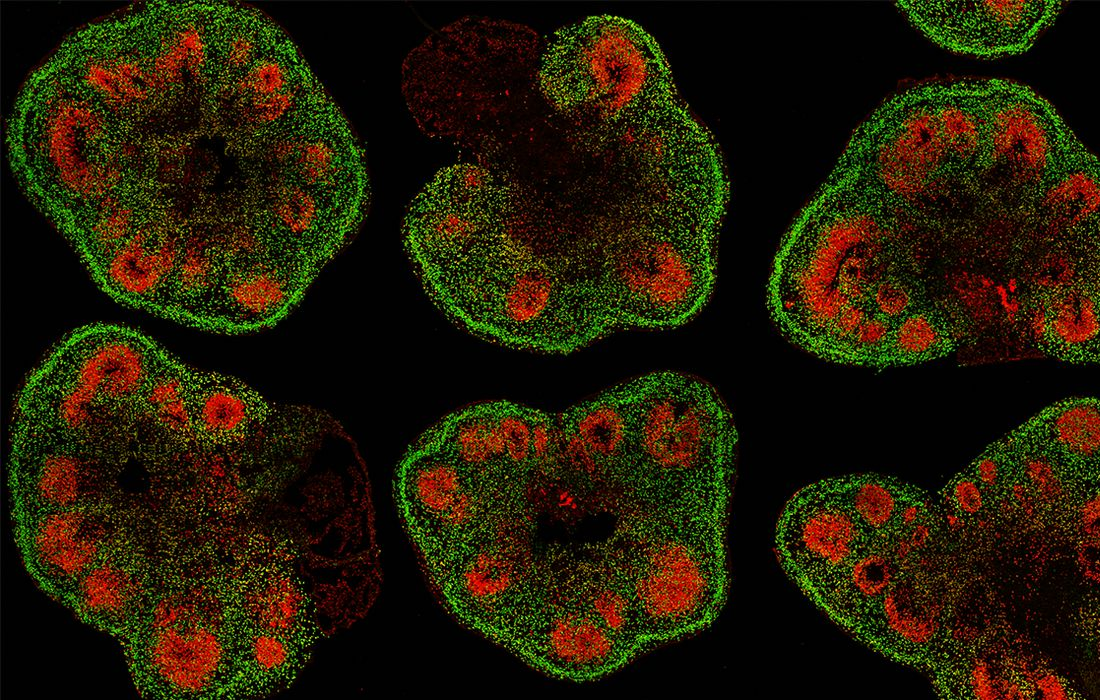
A team of engineers at UC Santa Cruz has developed a new method for remote automation of the growth of cerebral organoids
Cerebral organoids require a high level of expertise and consistency to maintain the precise conditions for cell growth over weeks or months. Using an automated system, as demonstrated in this study, can eliminate disturbance to cell culture growth caused by human interference or error, provide more robust results, and allow more scientists access to opportunities to conduct research with human brain models.
Autoculture also addresses variation that arises in organoid growth due to “batch effect” issues, where organoids grown at different times or at different labs under similar conditions may vary just because of the complexity of their growth. Using this uniform, automated system can reduce variation and allow researchers to better compare and validate their results.
Autoculture uses a microfluidic chip designed by the researchers, spearheaded by Associate Professor of Electrical and Computer Engineering Mircea Teodorescu and Biomolecular Engineering Ph.D. student Spencer Seiler. Their novel chips, created from a unique bi-layer mold, have tiny wells and channels for delivering minute amounts of liquid to the organoid, which allow the scientists to have a high level of control over nutrient concentrations and byproducts.
Unlike other methods for organoid growth which grow the cultures together in one dish, the Autoculture system contains a culture plate with 24 individual wells, so each well can be its own experiment in which cultures can be grown independently and fed liquids at varying, programmable concentrations and times. An in-incubator imaging system lets the researchers constantly remotely monitor organoid growth and morphology.
Additionally, a unique feature of the system is that feeding media for each individual culture can be pulled out for analysis at any point during an experiment. This allows researchers to non-invasively measure data such as pH and glucose levels which can be important for monitoring cell growth.
The microfluidics system is connected to the internet to allow scientists to remotely operate and retrieve real-time data from the system at any point, without disrupting the culture.
When measuring their cerebral organoids, the researchers found that the stem cells grown using the Autoculture system not only differentiated into various cell types normally, but actually looked healthier than those grown using standard methods.
SOURCE:
Spencer T. Seiler, Gary L. Mantalas, John Selberg, Sergio Cordero, Sebastian Torres-Montoya, Pierre V. Baudin, Victoria T. Ly, Finn Amend, Liam Tran, Ryan N. Hoffman, Marco Rolandi, Richard E. Green, David Haussler, Sofie R. Salama, Mircea Teodorescu (November 23, 2022). Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids. Scientific Reports.Retrieved from: https://www.nature.com/articles/s41598-022-20096-9