Regenerative Medicine News and General Information
New Nasal Vaccine for Alzheimer’s Enters Phase 1 Trial
Alzheimer’s disease is the most common type of dementia. It is a progressive disease beginning with mild memory loss and possibly leading to loss of the ability to carry on a conversation and respond to the environment.
In 2020, as many as 5.8 million individuals were living with Alzheimer’s disease in the US and the number of people living with the disease doubles every 5 years beyond age 65. This number is projected to nearly triple to 14 million people by 2060.
Scientists don’t fully know what causes Alzheimer’s disease, but there are several factors that are known to increase the risk of the condition. Some of them include:
- Age, which is the best known risk factor for Alzheimer’s disease.
- Family history.
- Researchers are studying whether education, dieta and environment play a role in developing the disease.
- There is also growing evidence that healthy behaviors, which have been shown to prevent cancer, diabetes and heart disease, may also reduce risk for cognitive decline.
There is currently no cure for Alzheimer’s, and treatments usually focus on helping people manage its symptoms.
New Phase 1 Trial
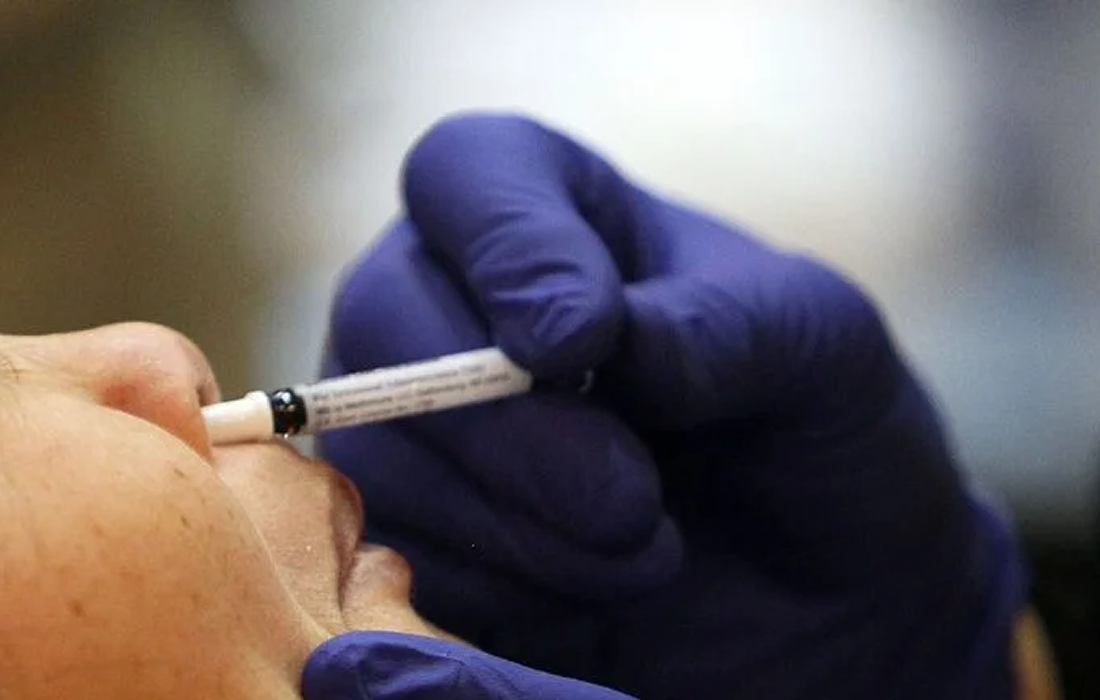
A new phase 1 trial of a nasal vaccine for Alzheimer’s disease is beginning. Scientists have used the vaccine with succes in a mouse model that simulates some of the characteristics of Alzhimer’s. If the new trial shows the vaccine to be safe in humans, further studies will test whether it is also effective.
The ongoing study is being developed by scientists from the Brigham and Women’s Hospital in Boston.
Dr. Howard L. Weiner, the lead research and co-director of the Ann Romney Center for Neurologic Diseases at the hospital, says, “The launch of the first human trial of a nasal vaccine for Alzheimer’s is a remarkable milestone.”
“Over the last 2 decades, we’ve amassed preclinical evidence suggesting the potential of this nasal vaccine for Alzheimer’s disease. If clinical trials in humans show that the vaccine is safe and effective, this could represent a nontoxic treatment for people with Alzheimer’s, and it could also be given early to help prevent Alzheimer’s in people at risk,” says Dr. Weiner.
The vaccine uses Protollin, an adjuvant for intranasal immunizations, to stimulate the immune system, which has been used in other treatments and has shown to be safe in humans. The researchers hope that it will activate white blood cells located in lymph nodes in the neck, encouraging these immune cells to clear beta amyloid plaques.
Investigators are going to initiate the phase 1 study, to determine the appropriate dosage and pharmacokinetics of the vaccine and if the results are positive move forward to phase 2 and 3 studies to determine the efficacy and safety of the treatment.
Sources:
Timothy Huzar (2021, Nov 24). Nasal vaccine for Alzheimer’s enters phase 1 trial. Medical News Today. Retrieved from:
https://www.medicalnewstoday.com/articles/nasal-vaccine-for-alzheimers-enters-phase-1-trial
https://www.cdc.gov/aging/aginginfo/alzheimers.htm
Image from: