Stem Cell Therapy for Specific Conditions
Stem Cell Changes in Rheumatoid Arthritis
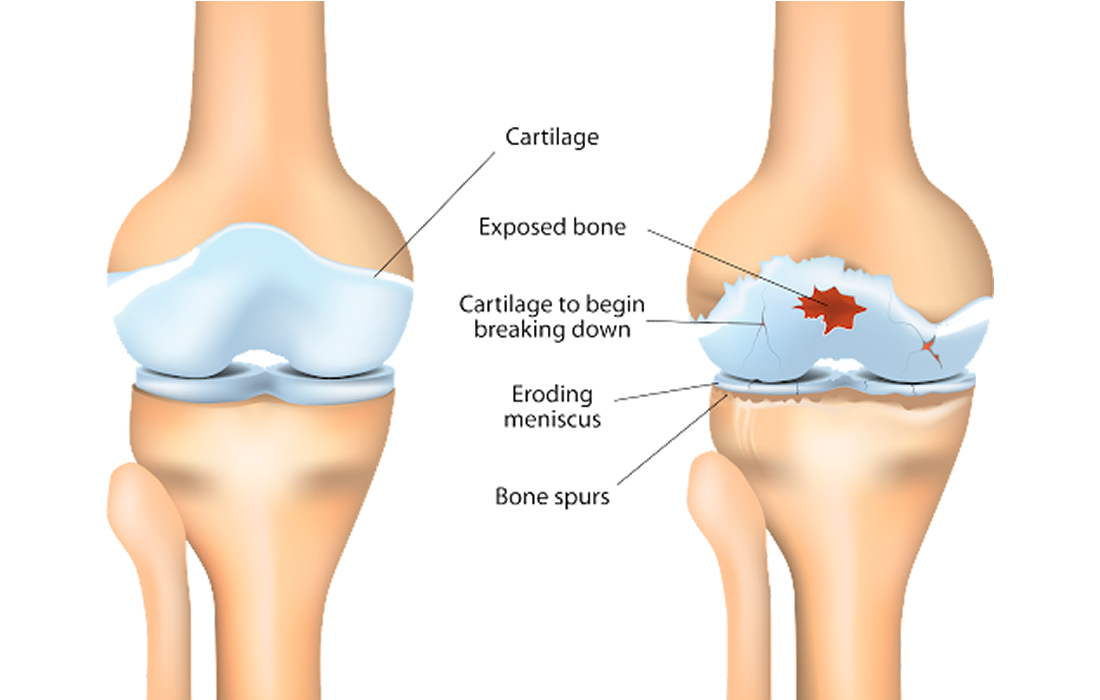
The onset of rheumatoid arthritis (RA) is associated with loss of systemic immunological self-tolerance, which results in the activation of autoreactive immune cells that target collagen-rich joint regions and present symptoms characterized as chronic, destructive joint inflammation.
As an autoimmune disease, there are limited curative options for RA that provide immunomodulation and articular cartilage or subchondral bone regeneration in the damaged joints. Mesenchymal stem cells (MSCs) can inhibit the immune response and are a potential candidate to treat various inflammatory autoimmune diseases, including RA. Once MSCs are exposed to proinflammatory cytokines in an inflammatory environment, they are activated and inhibit immune cell proliferation and activation by secreting anti-inflammatory cytokines.
The use of MSCs immodulation properties in RA patients is supported by the anti-arthritic potentials of MSCs observed in an RA animal model. Synovial fluid-derived MSCs (SF-MSCs) from RA patients are important for understanding disease pathogenesis and are easily obtained during the diagnosis or treatment of RA. These cells have enhanced immunomodulatory properties compared to bone marrow derived-MSCs in collagen-induced arthritis mouse models.
A group of researchers demonstrated the presence of SF-MSCs population in RA patients regardless of disease duration and also revealed that immunomodulatory properties of RA SF-MSCs are attenuated due to inflammation induced senescence in the inflammatory environment of RA joints. These immunomodulatory properties of RA-SF-MSCs are especially altered in an RA disease duration-dependent manner. Therefore, the results suggest that the therapeutic effects of disease affected MSCs on autoimmune diseases are highly associated with pathology and cellular environmental factors.
As in other autoimmune diseases, MSCs from patients with SLE exhibited increased frequencies of apoptosis (cellular programed dead), as evidenced by the downregulation of Bcl-2 and upregulation of Bax, and higher intracellular reactive oxygen species levels than those in normal MSCs.
Because MSCs modulate responses by both adaptive and innate immune systems, there is an interest in using MSCs to develop new cell-based approaches for the treatment of various inflammatory diseases. These immunomodulatory properties are not spontaneously acquired, they are initiated when MSCs are primed through exposure to pro-inflammatory cytokines and the secretion of anti-inflammatory factors that have inhibitory effects on immune cells. That is why mesenchymal stem cells can travel to areas where there is inflammation present.
Mesenchymal stem cells injected in mice models display reduced inflammation, ameliorated cartilage destruction, integration of injected MSCs into the synovium, decreased inflammation-induced systemic bone erosion, reduced osteoclast precursors, elevated anti-inflammatory cytokines and pro-inflammatory cytokine suppression.
Source:
Lee, HJ., Lee, WJ., Hwang, SC. et al. Chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid mesenchymal stem cells in rheumatoid arthritis. Stem Cell Res Ther 12, 502 (2021). https://doi.org/10.1186/s13287-021-02453-z