Stem Cell Therapy for Specific Conditions
Stem Cells Could Allow the Repair of Muscles Damaged by Diseases Such as Muscular Dystrophy
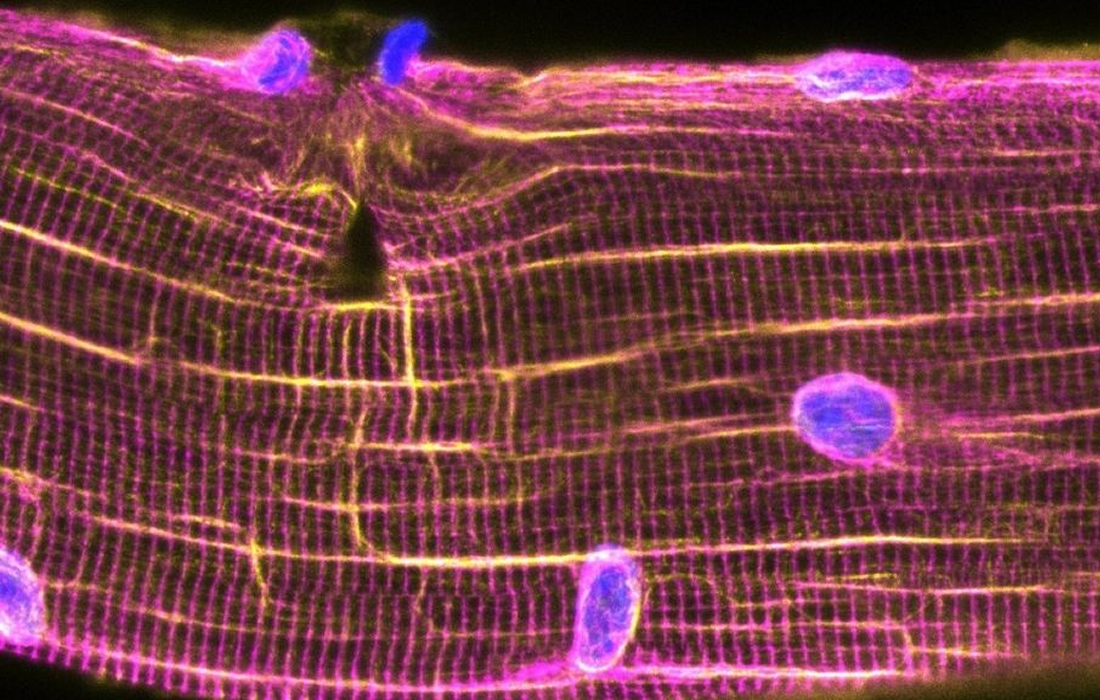
Therapies to target neuromuscular disorders affecting million of people worldwide are on the horizon thanks to research at the Montreal Clinical Research Institute of Montreal. Fusion of myoblasts, the stem cells responsible for the formation of skeletal muscles, could allow the repair of muscles damaged by diseases such as muscular dystrophy.
The formation of muscles, a complex process, requires the action of specialized cells, the myoblasts. In order for skeletal muscle to develop and regenerate, myoblasts must align with each other, move towards each other, and touch each other until their membranes are joined. This is called the myoblast fusion stage and is the basis for the formation of muscle fibers.
During embryogenesis, myoblast fusion is crucial, with mutations in certain genes resulting in the extremely rare clinical myopathy called Carey-Fineman-Ziter syndrome.
In adults, an army of satellite cells is responsible for muscle growth and regeneration. In response to activation signals, satellite cells proliferate, differentiate and fuse to repair damaged myofibers. The proteins and signaling pathways that control this fusion are still being identified.
Now a discovery made at the Montreal Clinical Research Institute of Montreal (IRCM) opens the door to the development of targeted therapies
In what was a key experiment, the researchers created a mouse model in which a protein involved in fusion is expressed in its active form in the mammal. During muscle development and regeneration, an increase in myoblast fusion was observed.
They also observed that this mouse model, when crossed with a mouse modeling limb-girdle muscular dystrophy 2B, can improve disease phenotypes.
The new data therefore provide direct evidence that the myoblast fusion process could be exploited for regenerative purposes and to improve the outcome of muscle diseases.
In the long term, this research shows, increasing cell fusion could “repair” muscles in other types of muscular dystrophy, such as Duchenne (occurring in 1 in 4,000 boys) or other severe conditions, such as cachexia (secondary muscle breakdown due to various diseases and some forms of cancer).
SOURCE:
Viviane Tran, Sarah Nahlé, Amélie Robert, Inès Desanlis, Ryan Killoran, Sophie Ehresmann, Marie-Pier Thibault, David Barford, Kodi S. Ravichandran, Martin Sauvageau, Matthew J. Smith, Marie Kmita, Jean-François Côté. Biasing the conformation of ELMO2 reveals that myoblast fusion can be exploited to improve muscle regeneration. Nature Communications, 2022; 13 (1) DOI: 10.1038/s41467-022-34806-4
IMAGE: https://cdn.mos.cms.futurecdn.net/r7BzEGM4QrT5Feo8TeXFXf.jpg