Regenerative Medicine News and General Information
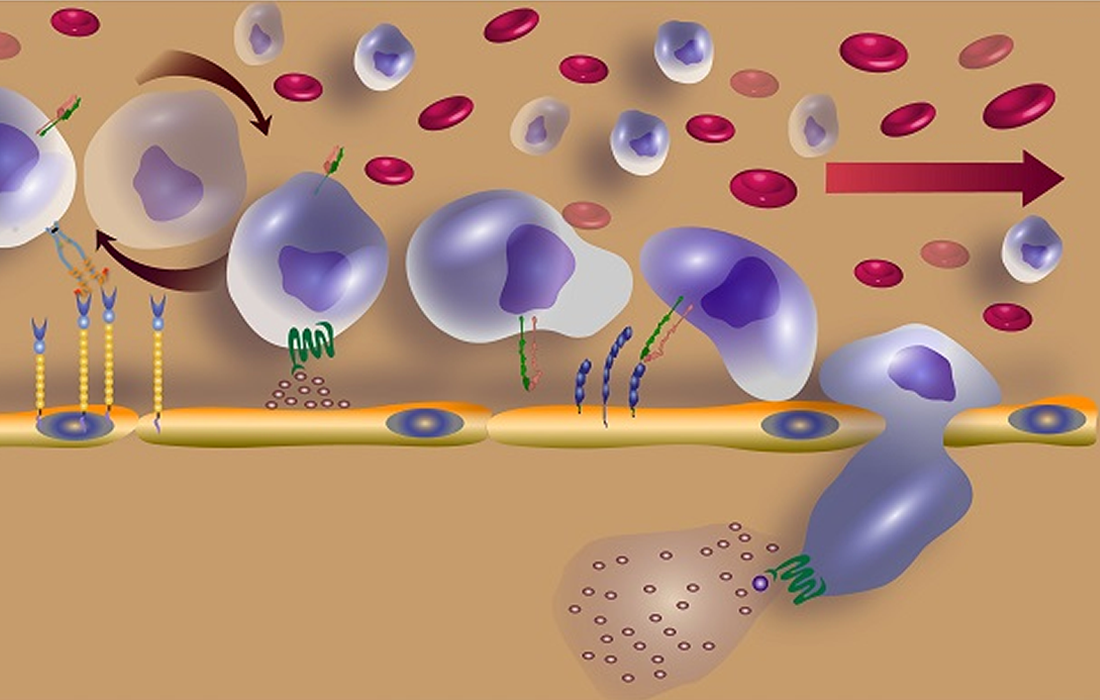
A New Way To Increase Stem Cells Homing Ability
Mesenchymal stem cells can be isolated from different tissues and organs and have been widely used in various medical fields, particularly regenerative medicine. The representative sources of MSCs are bone marrow, adipose, periodontal, muscle, and umbilical cord blood.
Depending on the source, differences have been reported, including their population in source tissues, immunosuppressive activities, proliferation, and resistance to cellular aging.
Bone marrow-derived MSCs (BM-MSCs) are the most intensively studied and show clinically promising results for cartilage and bone regeneration, but the isolation procedures for BM-MSCs are complicated because bone marrow contains a relatively small fraction of MSCs (0.001–0.01% of the cells in bone marrow). It is also a painful procedure.
In comparison adipose-derived MSCs (AD-MSCs) are relatively easy to collect and can produce up to 500 times the cell population of BM-MSCs. AD-MSCs showed a greater ability to regenerate damaged cartilage and bone tissues with increased immunosuppressive ability.
Stem cells derived from the umbilical cord (UC-MSCs) proliferate faster than BM-MSCs and are resistant to significant cellular aging.
New Study on the Use of MSCs Mimicking Nanoencapsulation
A new study published in the journal International Journal of Nanomedicine evaluated the use of MSC mimicking nanoencapsulations.
MSCs can regulate the inflammatory process and repair damaged cells in the different tissues. However, the functionality and migration ability of MSCs injected in vivo still show insufficient in some studies.
For that reason, MSC mimicking nanoencapsulations is an alternative strategy that does not involve using bioengineered MSCs.
MSC mimicking nanoencapsulations consist of MSC membrane-coated nanoparticles, MSC-derived exosomes and artificial ectosomes, and MSC membrane-fused liposomes with natural or genetically engineered MSC membranes.
This method retains the targeting ability of MSCs and has many advantages in terms of targeted drug delivery. These MSC mimicking nanoencapsulations are capable of encapsulating drugs with various components, including chemotherapeutic agents, nucleic acids, and proteins.
Some engineering and modifications that are possible include upregulation of receptors associated with chemotaxis through genetic engineering that can confer additional abilities of MSCs to home to specific sites, while the increase in engraftment maximizes the therapeutic effect.
Source:
Shin MJ, Park JY, Lee DH, Khang D. Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis. Int J Nanomedicine. 2021;16:8485-8507 https://doi.org/10.2147/IJN.S334298
Image from:
https://discovery.kaust.edu.sa/en/article/603/the-dynamic-molecular-changes-of-homing