Regenerative Medicine News and General Information
Why don’t we use Embryonic Stem cells?
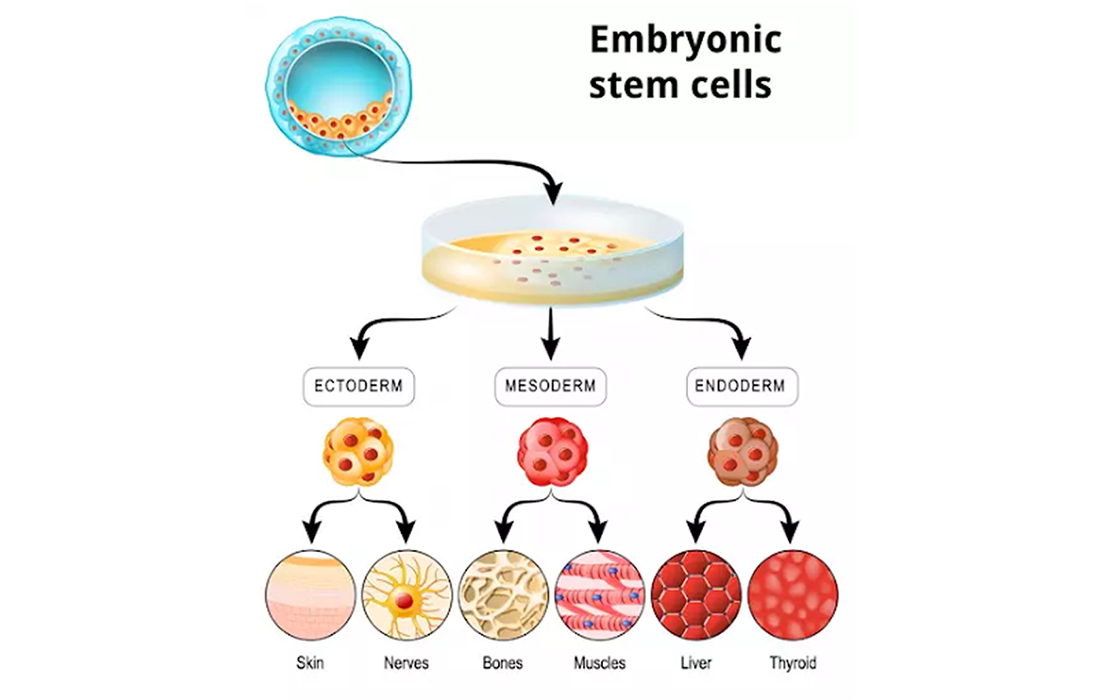
Stem cells are the body’s raw materials, cells from which all other cells with specialized functions are generated. Under the right conditions in the body or a laboratory, stem cells divide to form more cells called daughter cells. These daughter cells either become new stem cells (self-renewal) or become specialized cells (differentiation) with a more specific function, such as blood cells, brain cells, heart muscle cells or bone cells.
One type of stem cells are embryonic stem cells. These cells come from embryos that are three to five days old. At this stage, an embryo is called a blastocyst and has about 150 cells. These are pluripotent stem cells, meaning that they can divide into more stem cells or can become any type of cell in the body. This versatility allows embryonic stem cells to be used to regenerate or repair diseased tissue and organs.
These types of stem cells are obtained from early-stage embryos, a group of cells that forms when a woman’s egg is fertilized with a man’s sperm in an in vitro fertilization clinic. Because human embryonic stem cells are extracted from human embryos, several questions and issues have been raised about the ethics of embryonic stem cell research.
The National Institute of Health created guidelines for human stem cell research in 2009. The guidelines define embryonic stem cells and how they may be used in research. Also, the guidelines state embryonic stem cells from embryos created by in vitro fertilization can be used only when the embryo is no longer needed.
The embryos being used in embryonic stem cell research come from eggs that were fertilized at in vitro fertilization clinics but never implanted in a woman’s uterus. The stem cells are donated with informed consent from donors. The stem cells can live and grow in special solutions in test tubes or petri dishes in laboratories.
For embryonic stem cells to be useful in people, researchers must be certain that the stem cells will differentiate into the specific cell types desired. It has been discovered that there are ways to direct stem cells to become specific types of cells, such as directing embryonic stem cells to become heart cells.
Embryonic stem cells can grow irregularly or specialize in different cell types spontaneously. Researchers are studying how to control the growth and differentiation of embryonic stem cells. They might also trigger an immune response in which the recipient’s body attacks the stem cells as foreign invaders, or the stem cells might simply fail to function normally with unknown consequences. Researchers continue to study how to avoid these possible complications.
The type of stem cells that we currently use in our clinic are Umbilical Cord Mesenchymal Stem cells. They are derived from Warthon’s jelly umbilical cord tissue and obtained from full-term, normal C-section deliveries from consenting donors. These non-embryonic tissues undergo comprehensive medical, social and blood testing prior to procurement. After stringent screening the tissues are procured, processed, and tested in accordance with standards established by the American Association of Tissue Banks (AATB) and FDA requirements. We take every precaution to minimize potential risks of infectious disease testing and regenerative tests each step of its product along the way to ensure the highest quality safety standards are met and executed.
Umbilical Cord Mesenchymal Stem cells are easy to collect and process compared to other types of stem cells. There is no need to find a match donor because they are considered to be immunologically immature and lack some surface markers called Major Histocompatibility complex type II, thus they can evade the immune system, and there is a decreased risk of transmission of infectious diseases.
Research studies have also shown the advantage of these types of stem cells, like the higher number of stem cells available, higher concentrations of growth factors and cytokines. Also, there are no ethical issues with these types of stem cells. During the biologic acquisition, the babies are fine and the material donated and used is normally discarded.
Sources:
https://www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117